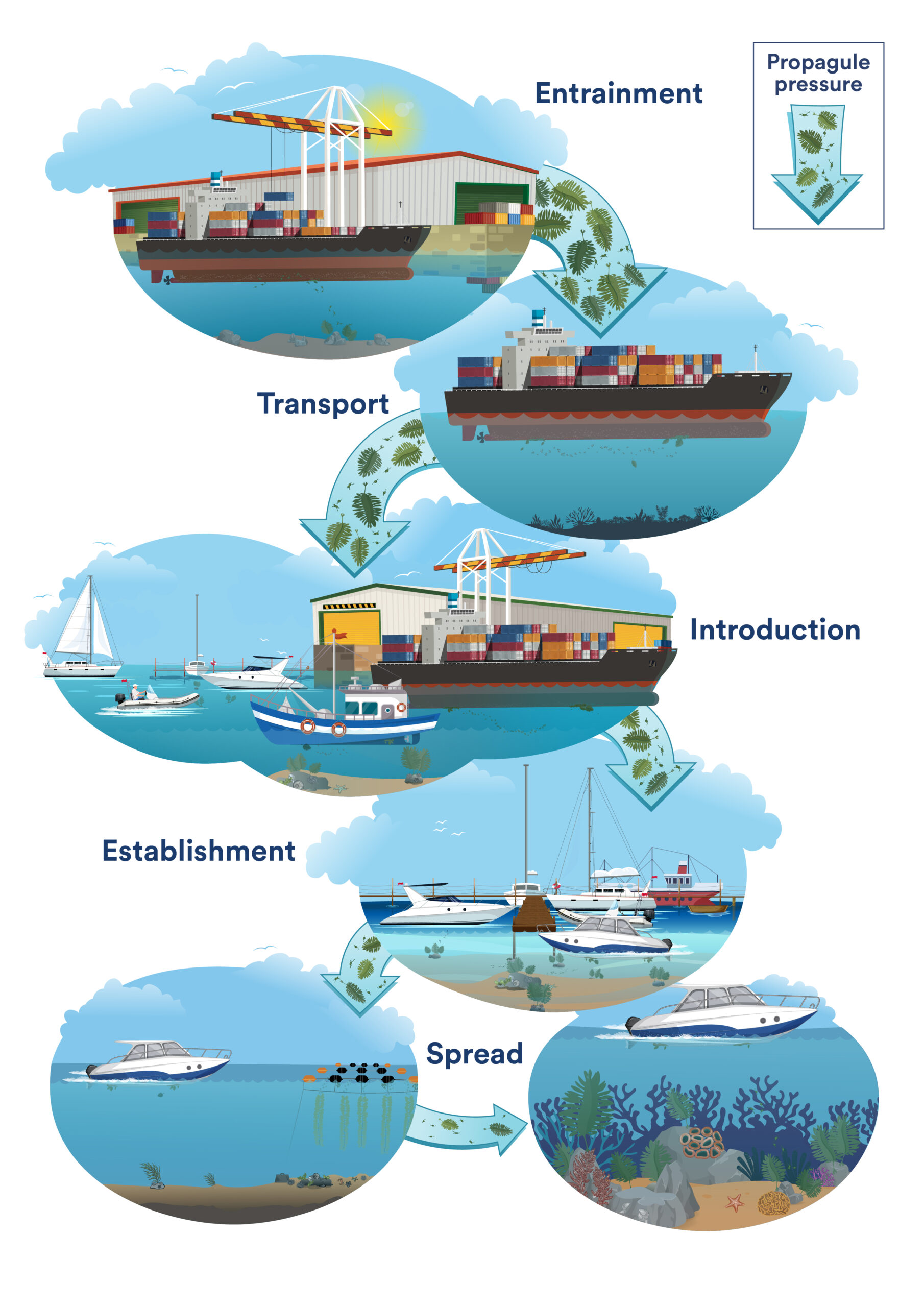
Last year over 700 boat owners who have non-trailered boats moored in marinas around Aotearoa…
The journey of eDNA marine intruder to the classroom
In line with Albert Einstein famous quote “if you can’t explain it to a six-year-old, you don’t understand it yourself”, the Detect team strongly believes that the rapid uptake of environmental DNA (eDNA) tools into New Zealand society will largely depend on our ability to effectively integrate this knowledge into schools’ curriculum. This vision was initiated last year with partner science teachers from Nelson College and Nelson College for Girls to explore how best to introduce research activities into the classroom. From there, Cawthron scientists have actively engaged with a range of schools and year groups to showcase a variety of marine biosecurity concepts and detection tools, including eDNA technologies (Figure 1).


Figure 1: (Top picture) Dr Anastasija Zaiko describing marine biofoulers on settlement plates (Nelson College for Girls, December 2020). (Bottom picture) Drs Anastasija Zaiko, Ulla von Ammon and Xavier Pochon sharing their passion for eDNA detection tools with hundreds of kids during Seaweek (Nelson Intermediate, March 2021).
Most recently, the team had a unique opportunity to collaborate with teacher Johnnie Fraser at Nelson College as part of a science extension class involving year 9 (~13-year-old) students. During three dedicated sessions spread over a school term, Scientists from Cawthron and University of Otago brought eDNA technologies right into the classroom. The first session consisted of extracting DNA and RNA from the tunicate Styela clava, the Mediterranean fanworm Sabella spalanzanii and eDNA from an aquarium using the Pretty Damn Quick Extractor (PDQeX, MicroGem) instrument (Figure 2)




Figure 2: The first session focused on extracting DNA and RNA from two notorious marine invasive species (Nelson College, November 8, 2021).
During the second session, students worked in small groups to prepare their very first quantitative Polymerase Chain Reaction (qPCR) analysis using the DNA samples extracted during the first session (Figure 3). They used specific primers and probes to measure the quantity of S. spallanzannii and S. clava in their samples using the MyGo Mini qPCR instrument, which produced results in about 30min.




Figure 3: The second session was dedicated to qPCR analyses using the MyGo mini-PCR instrument (Nelson College, November 10, 2021).
The third and last session consisted of sequencing eDNA samples using a Oxford Nanopore MinIon DNA sequencer (Figure 4). Prof. Jo Stanton gave a brilliant introduction on Nanopore sequencing technology. The students witnessed high-throughput sequencing in real time and learned to load ‘test’ MinIon flow cells.




Figure 4: The third session was all about DNA sequencing using MinIon Nanopore technology (Nelson College, November 17, 2021).
This research exchange was mutually beneficial. Scientists were able to improve their protocols and adapt their teaching to maximise learning and engagement levels. Students were exposed to new knowledge and technologies that are otherwise not accessible to them, got inspiration from the “real-world” scientists and took the challenge to capture this experience and communicate it to the world – here is the video:
Our continued ambition is to inspire students and offer them a platform where they can naturally engage, learn, and communicate important issues that are directly relevant for preserving our coastal ecosystems for future generations of New Zealanders.



Comments (0)